Research in Day-to-day Practice
Dr. Amit Nakanekar
MD (Ayu.), Ph.D., Assistant Professor,
Department of Kaychikitsa, Government Ayurveda College, Nagpur
Clinics or practice is routine part of our day-to-day life. Although many of us are interested in carrying out some kind of research. Still we may have many doubts, queries and problems regarding the research that can be formulated from day to day practice. So, here we are trying to cover the aspects of research those are concerned with day to day practice.
Image-1 Villagers are carrying the patient of a scorpion bite to PHC

In the given picture villagers are carrying the patient of a scorpion bite to a primary health care centre. Here patient is suffering from poisonous scorpion bite that can cause death of the patient. Another picture is of Dr.Himmatrao Bawaskar who has discovered the fact that scorpions bite can be fatal to cause a death. He observed this fact while working as Medical Officer in a PHC (primary health care centre) of Konkan area of Maharashtra. This area of Maharashtra is considered as a very remote place with lack of basic facilities like connectivity, transport and many more. In spite of facing lots of adverse conditions while working in a PHC, he found out the fact that a poisonous scorpion bite can kill a patient and treatment to reduce death rate. So ‘Necessity is Mother of Invention’. You may go through some of these publications by Dr.Himmatrao Bawaskar.
Image -2 publications by Dr.Himmatrao Bawaskar

He has established a fact of pulmonary oedema after scorpion sting. A Lancet publication by him shows cardiac premonitory signs & symptoms of red scorpion sting. Later he established the treatment in the form of tablet ‘Prazocin’ by observing its role in prevention of pulmonary oedema and decrease in death rates. It is praiseworthy to note that all these publications came from him, while he was working in a remote area of Maharashtra. [1]
Another remote area in Maharashtra is Gadchiroli. It is a very remote, densely forested, underdeveloped area, and mostly affected by terrorist’s activities. Higher incidence of neonatal mortality was one of the problem of community leaving in this area. There is a couple named Dr. Abhay Bang & Dr. Rani Bang who found out some innovative and simple thought called ‘Kangaroo’ method. In spite of having a lot of poverty & lack of the instruments and advanced gadgets, out of reach to new world; neonates and children of residential community can be raised safely by adopting this simple method. This ‘Kangaroo’ method established by them later on accepted by WHO. This couple published more than 500 research articles while just sitting in Gadchiroli area of Maharashtra. WHO has released a statement for adoption of ‘Kangaroo’ method to reduce neonatal mortality. Even this couple contributed a lot in other fields related to neonatal mortality and child hood pneumonia. So it is meant that, it is false to suggest that medical breakthroughs come only through government research. [2]
Image 2. publication by Dr. Abhay Bang and Dr.Rani Bang:
What is Relationship between Research and practice?
Good research is necessary to improve our practice. While good practice & good observation in our practice can lead to some good research which in turn is responsible for improvement in practice. A good practice and good research both are interdependent. It means that there is impact of good research on a practices i.e. good research can bring-out some new medicine, new technology, new adaptation, new method that will help to improve practice. Good practice along with good observations also can generate very good research question. Therefore we have to keep balance between good research and good practice .So that we will continue to do both good research as well as good practice.
In this lecture we will learn about ‘Reverse Pharmacology’, Case Studies and Case Reports, unusual findings, various Clinical Studies and ‘Evidence Based medicine’.
How research can be done in practice?
Reverse Pharmacology:
Dr.Ashok Vaidya has done some remarkable things. He is a pioneer in establishing fast track of reverse pharmacology for research in Ayurveda. In a conventional pharmacology, the way for development of drug is from benches to beds. The sequential pattern includes establishment of Molecule → Pre-Clinical Studies → Animal Studies → Market.
It is a quiet expensive and time consuming. While in case of Ayurveda, we are already using the medicine in our clinical practice, therefore by following reverse order i.e. from clinics to benches we can find out the things/fact that were resulted in the form of efficacy. Dr.Ashok Vaidya sir has been established this approach known as ‘Reverse Pharmacology’. [3]
Image 3. The format for clinics to laboratory
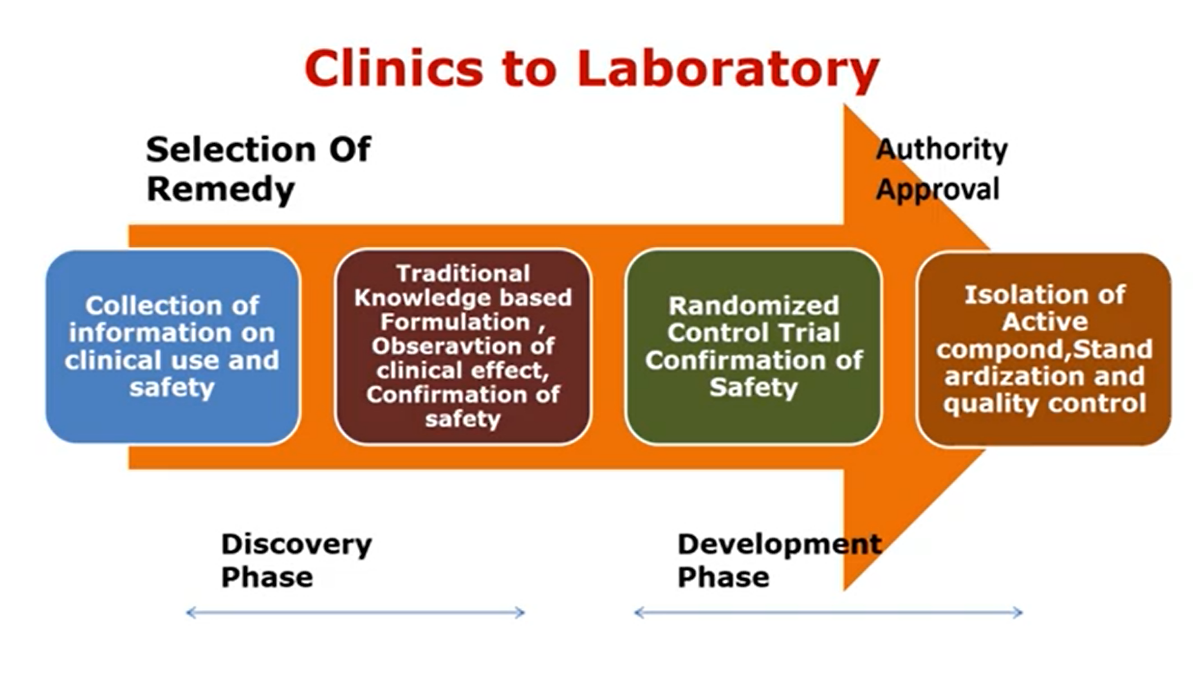
So this is an exact mechanism from ‘Clinics to Laboratory’. In case of Ayurveda research we can follow similar approach i.e. from ‘Clinics to Laboratory’. Because in routine Ayurvedic practice many of formulations are already in use.
Various types of studies –
What are the different type of clinical studies that we can do in our day to day practice & our day to day clinical set up?
- Descriptive study: – This is a type of study where clinician can describe a kind of patient, observations or experiences without comparing to any group. The researcher, clinicians interact with the participant through survey or Interviews to collect necessary data or information.
- Analytical study – This type of study needs comparison group e.g. comparison between normal and diseased individuals, comparison between alcohol addicted and non-addicted individuals.
Descriptive study has two types –
A) Personal level individuals study :
1. Case reports: It includes individual case report consisting of some new or interesting findings and treatment. Though case report is first level of evidence, it may help while treating the patients. Similarly it can provide some important leads to the science to manage tricky situations or it can provide some kind of innovation.
2. Case series: Reporting of case series of non-specific pneumonia has been brought attention on the emergence of the new disease. Later on this disease term as COVID-19 by WHO. This case series is responsible to bring some important breakthroughs to our day to day practice. The emergence of COVID-19 has be identified by Case series itself.
3. Cross sectional study: It is single examination study. Identifying risk factor is the first objective of this study. It is also very useful to find the prevalence rate of various diseases. [5]
4. Surveillance study
B) Aggregate level population type study: It includes ecological correlation study:
Algorithm for classification of clinical research? –
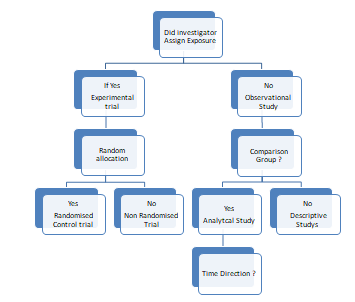
Did investigator has any exposure? If it is yes-then experimental trial and if it is no- then observational study.
In observational study, investigator do not implement any kind of intervention however he/she will observe the group in curious way or detail way. If an observational study has comparator group then it will be analytical study.
Example – Observation among the groups of alcoholic and non-alcoholic individuals. If we want to study whether alcohol can cause cirrhosis of liver? Individuals going to be observed can be allocated like,
Group A. Individuals consuming alcohol and having cirrhosis.
Group B. Individuals not consuming alcohol but having cirrhosis of liver.
Group C: Individuals consuming alcohol but not having cirrhosis of liver.
By observing these three groups we can perform an ‘Analytical study’. When there is no comparison group then it will be ‘Descriptive study’. If there is a random allocation for experimental studies then it will become a ‘Randomized Clinical Trial and no random allocation then it will become a ‘Non-randomized clinical trial’.
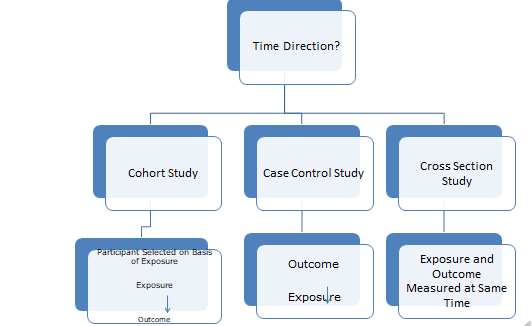
There is time direction for ‘Analytical studies’. The three types are
1) Cohort study
2) Case Control study
3) Cross sectional study
Case control study: In this type of study, method used is from outcome to exposure e.g group of individuals having disease with or without prior exposure to particular substance / factor will be considered as ‘Case’. While very similar individuals having no disease with or without prior exposure to particular substance /factor will be considered as ‘Control’.
Cohort study: In case of cohort study which usually follow a participants selected on the basis of exposure e.g. average duration for onset of liver cirrhosis can be predicted by observing the persons who are taking alcohol very often.
Cross section study: Exposure and outcome is measured at same time.
Case report and Case series
Case report includes single case. While case series includes more than one patient that has been reported by researcher. Case report is simplest study design and very weak evidence, yet it gives unusual thought provoking phenomena. Investigators are often practicing clinicians. Therefore it is very important to record a case in a good way or to report a case series as far as practicing clinicians are concerned. Case reports & case series has ability to generate a research question and also gives answer for that research question. If you don’t ask research question you will never get answer.
Case report- CARE TEMPLATE
Case reports has to be published after following specific guideline called as ‘CARE’ guideline. CARE guideline are initially published in 2013 in Journal of epidemiology under leadership of Dr David Riley. This guidelines gives a specific template and sequence for publication of case report.
General considerations:
Ensure that all patient data has been DE identified and obtained the necessary approval, consent or consent from of hospital. Mention that you don’t have any competing or conflict of interests.
Title – It should include word “Case Report.’’
The title should be sufficient enough to describe greatest interest of the author. This could be way to present the diagnosis, test results, the interventions or the outcome.
Abstract – It should be written in about 200 words. Summarize the following information if relevant
1) Rationale for this case report
2) Presenting concerns of the patient (such as chief complaints or symptoms, diagnosis)
3) Interventions (such as diagnostic, preventive, prognostic, therapeutic exchange)
4) Outcomes
5) Main lessons to be learn from this case report.
Key words – Provide 2 to 5 key words that will help readers to search and find this case report.
Introduction – Briefly summarize the background and context of this case report
Presenting concerns – Describe the patient characteristics (such as the relevant demographics – age, gender ethnicity, occupation) and their presenting concerns with relevant details of related past interventions
Clinical findings – Describe the
(1) Medical, family & psychological history including lifestyle & genetic information
(2) Other pertinent co-morbidities interventions (other therapies including self-care)
(3) The physical examination (PE) focused on the important findings including results from testing
Timeline- create a timeline that includes specific date and times in a table, figure, or graphic. Visit www.care statement org / case report example, for one example of a ‘Case report’ timeline.
Diagnostic Focus & Assessment- provide an assessment of the
1) Diagnostic methods (including laboratory testing, imaging results, questionnaires, referral diagnostic information)
2) Diagnostic challenges (such as limited ability to complete an evaluation, patient availability, cultural)
3) Diagnostic reasoning including other diagnosis considered
4) Prognostic characteristics (such as staging in oncology)
Therapeutic focus & Assessment –
1) Types of Intervention (such as pharmacologic, surgical, preventive, lifestyle, self-care)
2) Administration & intensity of the intervention (including dosage, strength, duration, frequency.)
Follow up & Outcomes – describe the clinical course of this case including all follow up visit as well as
1) Intervention modification, interruption or discontinuation and the reasons
2) Adherence to the intervention and how this was assessed.
3) Adverse effects or unanticipated events
Also describe. –
1) Patient reported outcomes
2) Clinician assessed and reported outcomes
3) Important positive & Negative, test results.
Discussion –
Describe the strength and limitations of this case report including case management, the scientific and medical literature related to this case report. Discuss the rationale for your conclusion potential causation & the ways this case might be generalized to a larger population. Finally main findings of this case report and ‘take away’ messages should be written.
Patient Perspective –Wherever appropriate the patient should share their experience of their care in a narrative published within this case report or accompanying this case report.
Informed consent – Ensure that the patient provided there Informed consent for the publication of this case report.
Examples of published case study in PubMed-
1) Guillian Barre syndrome associated with COVID -19 infection – a case report. [4]
2) An Ayurvedic approach in the management of Guillian Barre syndrome – A case study [5]
3) Analysis of Virechana karma with Danti avaleha A retrospective study – case series published in Journal of Ayurveda & Integrity medicine.[6]
This type of case series can be published by any Vaidya (Ayurveda clinician) or clinicians from Kayachikitsa branch those are dealing with such type of cases in their day to day clinical practice. This type of case series can give boost to improve the clinical practice.
Guidelines of various types of studies
- Search a website Equator network.org, to find guidelines for various types of studies.
- CONSORT Guideline – RCT (Randomised controlled trial)
- STROBE Guideline – observational study
- PRISMA Guidelines- systematic review
- SPIRIT Guidelines -study protocol
- STARD Guidelines- diagnostic/ prognostic studies
- CARE Guidelines-case report
- AGREE Guideline – clinical practice guideline
- SRQR Guidelines- qualitative research
- ARRIVE Guideline- Animal pre- clinical studies.
- SQUIRE Guidelines- quality improvement studies
- CHEERS Guideline -economics evaluation
CONSORT guide line should be followed for performing various clinical trials. Image given below depicts CONSORT flow diagram. While performing clinical trial, it is essential to solve CONSORT statements (25 item checklist) and also mention flow diagram. Visit to CONSORT Guideline, the CONSORT statement website & go through the details of how to follow. [7]
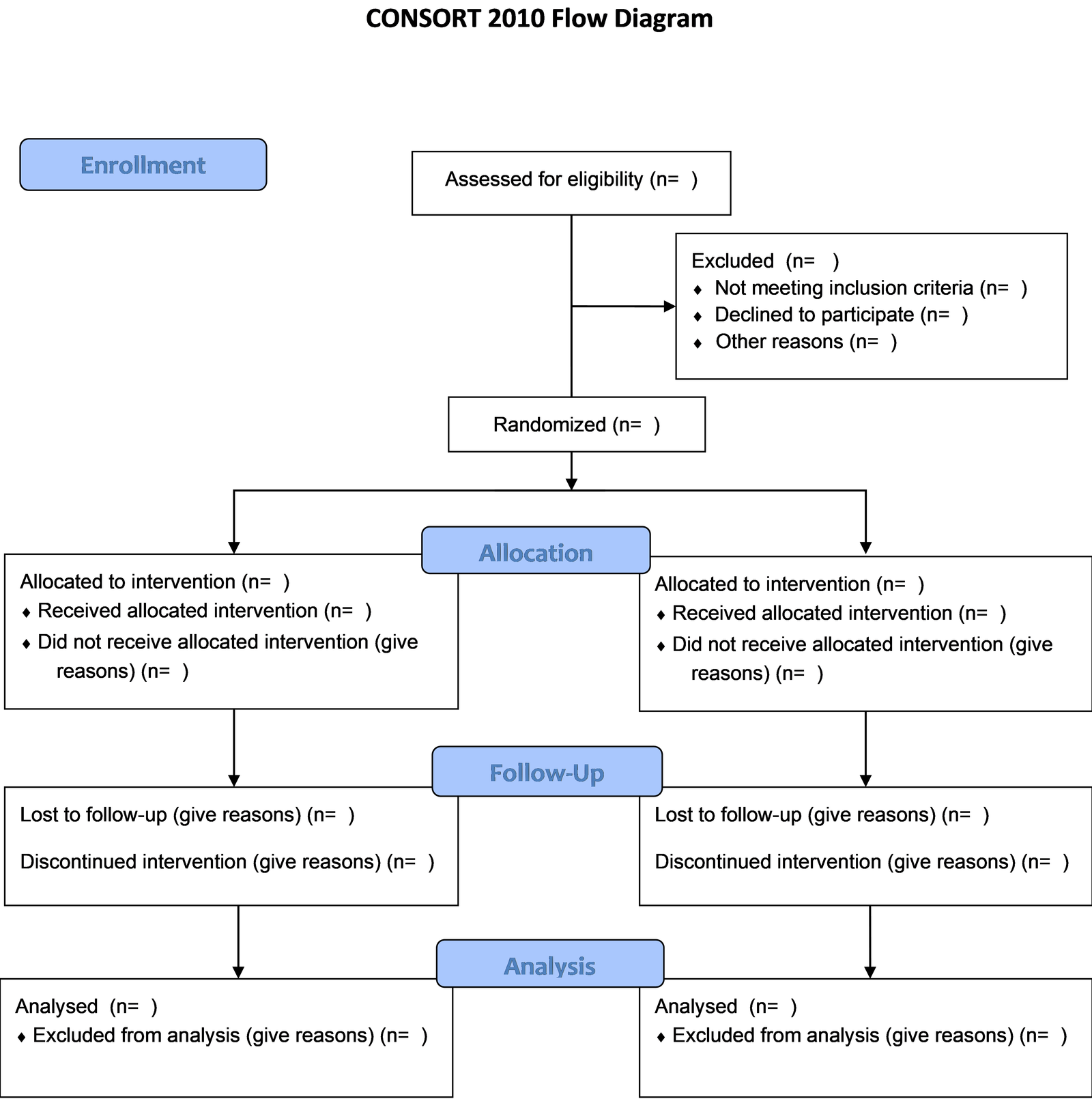
Registration of Clinical Trial is mandatory –
Before performing clinical trial, its registration is mandatory. Before recruiting any subject of your clinical studies, you have to register for CTRI. (Clinical Trial Registry India)
Unusual clinical findings-
Some unusual clinical findings has to be reported. Here two examples of treated case from our hospital has been displayed. First example is pseudohermaphroditism. In this patient’s clitoris is looking like a glans penis, eventually it was diagnosed as late onset adrenal hyperplasia. We treated this case with Ayurvedic management and presently patient is living very nice life. Another example is of purple colour urination. Snaps can be used for reporting unusual findings.
Standardization clinical procedures-
One of the area providing research opportunity to Ayurveda clinicians could be ‘Standardization of clinical procedures’. Automated instruments can be developed for the purpose of standardization of clinical procedures. Development of standard instrument and outcomes derived through it has to be published.
E.g. Auto enema apparatus, Naditarangini instrument (नाडी तरंगिणी). Data generated through instruments can be utilized for publication purpose.
Evidence Based Medicine:
Impact of good research on practice can be learned through EBM.(Evidence Based Medicine) The physician who knows EBM better can do the practice in much better way. Guyatt firstly mentioned EBM in 1991. It is about integrating individual clinical expertise with best available clinical evidence derived from systemic research. It should be conscience, explicit and judicious use of current best evidence in making decisions about individual patient.
e.g .How can a patient of particular disease will get the best treatment plan based on recent research? You can do PubMed search based on evidence available and after matching those evidences with actual patient data, you can select the best treatment to be offered to the particular patient in that particular scenario. Such type of integration is called as evidence based medicine. When you are educated you will believe on only half of what you hear and read. But when you are knowledgeable and intelligent enough, you will know which half to believe & which half not to believe.
Hierarchy of Evidence Based Medicine
Where we can find components of evidence based medicine?
1) Cochrane Data Base
2) Other Meta-Analysis.
3) Randomized control Blinded trials
4) Randomized control unblinded Trial
5) Randomized uncontrolled Trial
6) Non Randomized controlled trial
7) Publications on www.rhlibrary.com
8) Observational studies.
9) Review Articles.
10) Cross sectional study
11) Editorials
12) Clinical practice guidelines
13) Electronic database like DHARA, Pubmed, Ayush portal
14) Clinical trial registries
15) Monographs and textbook
16) Internet sources
Level of Evidence:
Level A- Good scientific evidence. You must discuss this service/investigation /treatment with eligible patients. Risk to benefit is more.
Level B- Fair scientific evidence
Level C- Fair scientific evidence but balance between risk to benefit is narrow
Level D- Fair Evidence suggests that risk > benefits
Level E- Scientific evidence lacking or Poor. Clinicians should help patient to understand uncertainty.
Limitations of Evidence Based Medicine-
1) Characteristics of patients to RCT may be different than the patient which physician is confronted e.g., – Race, Genetics, Culture, Environment.
2) Clinicians may have specific query regarding some aspects which could be too narrow to be assessable by studies.
3) Besides there are no RCT to support or to reject half of clinicians do in patient care and take decisions.
4) Patient may choose less effective therapy.
Obstacles in Evidence Based Medicine
1) Ignorant, Reluctant, Medical Representative Dependent clinicians – do not spend time in reading actual evidence.
2) Pharma companies
3) Scarcity of evidences – maximum up to 35% medical care is based on RCT
4) Patients ignorant and prejudices mind set.
How to overcome the limitations?
1) Try to read evidences and stay updated.
2) Follow ‘Good Clinical Practice’ guidelines.
3) Try to analyse medical representative’s report in scientific way.
4). Don’t forget, all of us who treat patients every day will be become patient someday…
WHY – Research is to see what everybody else has seen, and to think what nobody else has thought. (Albert – Szent-Gyorgyi)
| Multiple choice question
1) Who played pivotal role in establishing fast track of reverse pharmacology? (a) Dr. Himmat Rao Bawaskar (b) Dr. Ashok Vaidya (c) Dr. Abhay Bang (d) Dr. Rani Bang 2) Some normal individuals addicted to something and some are not addicted to that, if we consider occurrence of particular disease with that addiction What type of observational study is this? (a) Descriptive study (b) Analytical study (c) Surveillance study (d) None of the above 3) Which study is useful to find prevalence rate of various diseases? (a) Cohort (b) Case series (c) Cross sectional (d) Ecological correlation 4) In which of following study participants are selected on the basis of exposure? (a) Cohort study (b) Case control study (c) Cross sectional study (d) Case report 5) Which of following guidelines has to be followed for carrying out qualitative research? (a) STROBE (b) SQUIRE (c) STARD (d) SRQR 6) In which level of Evidence, scientific evidence is lacking or poor, therefore clinicians should help patient to understanding uncertainty. (a) Level E (b) Level D (c) Level C (d) Level B 7) Level B is ……………………. (a) Fair Evidence suggests that risk > benefits (b) Fair scientific evidence but balance between risk to benefit is narrow (c) Fair scientific evidence (d) Good scientific Evidence should discuss service with Eligible patients. 8) Which of the following is limitations of EBM? (a) Ignorant, reluctant, Medical Representative dependent clinicians (b) Characteristics of patients e.g., – Race, Genetics, Culture, Environment. (c) Pharma companies (d) patients ignorant and prejudices mind set. 9) Who published Care Guidelines? (a) David Riley (b) David Shaw (c) David Baltimore (d) David Deming 10) Which of the following is impact of Good Clinical research on practice? (a) some new medicines, (b) some new technology, (c) some newer adaptation (d) all of above |
| Question no | Answer |
| 1 | b |
| 2 | b |
| 3 | c |
| 4 | a |
| 5 | d |
| 6 | a |
| 7 | c |
| 8 | b |
| 9 | a |
| 10 | d |
References-
1. Kale A. A. (2012). A crusade against scorpion sting: life and works of dr. Himmatrao bawaskar. Journal of family medicine and primary care, 1(1), 52–55. https://doi.org/10.4103/2249-4863.94453
2. Bang A. T. (1991). Pneumonia and Child Mortality in India. The National medical journal of India, 4(4), 157–158.
3. Patwardhan, B., & Vaidya, A. D. (2010). Natural products drug discovery: accelerating the clinical candidate development using reverse pharmacology approaches. Indian journal of experimental biology, 48(3), 220–227.
4. Randhawa, J., Randhawa, H. S., & Toor, P. (2021). Pharyngeal-Cervical-Brachial Variant of Guillain-Barré Syndrome in a Patient of COVID-19 Infection. Cureus, 13(9), e17945. https://doi.org/10.7759/cureus.17945
5. Nakanekar, A., Bhople, S., Gulhane, H., Rathod, S., Gulhane, J., & Bonde, P. (2015). An ayurvedic approach in the management of Guillain-Barre syndrome: A case study. Ancient science of life, 35(1), 52–57. https://doi.org/10.4103/0257-7941.164540
6. Chaganti, S., & Prasad, B. S. (2015). Analysis of Virechana karma with Danti avaleha: A retrospective study. Journal of Ayurveda and integrative medicine, 6(4), 300–304. https://doi.org/10.4103/0975-9476.172420
7. http://www.consort-statement.org/consort-statement/flow-diagram accessed on 20 October 2020





